By
Karla Adam,
Lateshia Beachum and
Carolyn Y. Johnson
June 16, 2020 at 5:50 p.m. CDT
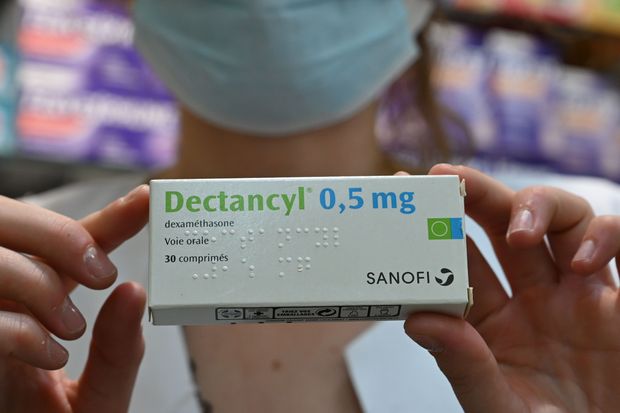
LONDON — An inexpensive steroid reduced deaths from coronavirus infection, helping some of the sickest patients with severe lung damage survive the illness, according to British clinical trial results announced Tuesday in a news release.
The 60-year-old drug, dexamethasone, is the first medication shown to increase people’s chances of surviving covid-19, the disease caused by the novel coronavirus. It reduced the risk of death for patients on ventilators by a third and cut the risk of death for patients on oxygen by a fifth, heartening news that drew widespread interest and hope.
British regulators speedily approved the drug for use in hospitalized patients requiring oxygen, and Prime Minister Boris Johnson trumpeted the results, declaring there was “genuine cause to celebrate a remarkable British scientific achievement and the benefits it will bring not just in this country but around the world.”
AD
ADVERTISING
Dexamethasone is a workhorse steroid typically used to treat inflammation, including flare-ups of rheumatoid arthritis, and was given as a tablet, liquid or as an intravenous preparation in the trial. Some other steroids are also being tested against covid-19.
Outside physicians tempered their optimism about the news with urgent calls to release details of researchers’ findings so doctors could pore over the data and understand the benefits and limitations of the drug.
“If this is reproducible and if it pans out, it’s a huge win — but that’s a lot of ‘ifs,’” said Isaac Bogoch, an infectious diseases specialist at Toronto General Hospital. “It’s cheap as borscht, as my grandparents would say. It’s widely available. Every single physician on the planet that practices hospital-based medicine is comfortable using this drug.”
But without complete data from the trial, physicians such as Bogoch are in a difficult predicament. Bogoch said that if a patient came into the hospital today with covid-19, he is not sure whether he would use the drug or when he would start it without more information. As of Tuesday afternoon, treatment guidelines from the World Health Organization and the National Institutes of Health recommended against using steroids because of the lack of evidence that the benefit of the drugs outweighs potential harm.
Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in an interview Tuesday that a group of experts who craft guidelines on treating covid-19 is already planning to meet to evaluate the data and assess whether recommendations should be changed.
“That’s the whole purpose of the guidelines panel, which can meet on the drop of a dime,” Fauci said.
AD
The results come from a large British clinical trial, designed to test six potential therapies, including dexamethasone, against typical care. The British investigators leading the trial said they chose the unconventional route of stating the results before providing additional data because of the public health importance.
The investigators estimated that dexamethasone could prevent one death in about eight ventilated patients or one death in about 25 cases in which patients required supplemental oxygen.
Peter Horby, the University of Oxford professor who led the trials, joined Johnson at a news conference at 10 Downing Street. He said that of the six drugs studied during the past three months in the largest covid-19 clinical trial in the world, dexamethasone was “the one I’m most excited about.”
“The doctors can walk across to the pharmacy cupboards in five minutes and they can prescribe it. They know how it works, and they know it’s going to help. And so it can be done this evening, it’s fantastic,” Horby said.
Chris Whitty, England’s chief medical officer, said on Twitter that it’s “the most important trial result for covid-19 so far.”
But to physicians, it is yet another example of the difficulty of practicing medicine during the covid-19 era, in which scientific findings are routinely shared by news release, media report or preliminary studies that are not yet subjected to peer reviews that help identify shortcomings or flaws.
“I think that doctors are generally not going to change practice on a press release, no matter how promising it seems,” said Nahid Bhadelia, medical director of the special pathogens unit at Boston Medical Center.
AD
A news release of interim data from the early safety trial of a vaccine being developed by the biotech company Moderna sent the stock market soaring, but scientists are still waiting to see the complete results. On Monday, a controversy about hydroxychloroquine, an anti-malarial drug that was promoted by President Trump and others on preliminary evidence, appeared to be resolved when the Food and Drug Administration withdrew an emergency-use authorization — partially because of data from the same British trial that showed that drug did not help patients.
The British investigators leading the trial randomly assigned patients into groups that received dexamethasone or regular care. More than 2,000 patients received low doses of dexamethasone once a day for 10 days, while 4,000 patients received typical care.
Outside physicians said it was important for people to understand that this was not a drug for people with mild symptoms or a cure for covid-19. The effects were seen in patients with severe lung damage — requiring supplemental oxygen or a ventilator.
AD
Instead of targeting the virus, steroids are aimed at the body’s immune response — which can rage out of control as people fight off an infection, causing inflammation and lung damage. The logic behind testing steroids in people is that the drugs could quell inflammation in the lungs or other components of the immune response.
The use of steroids against coronaviruses had been a source of medical debate, with some worrying that the drugs could be a risk factor for infection. Steroids, including dexamethasone, have been tried with mixed results against acute respiratory distress, a form of lung failure that bears some resemblance to severe covid-19 infections.
“We always have a concern when we give steroids to patients in general, because steroids depress the immune system,,” Bhadelia said. “They carry the risk of making the current infection worse and increase the chance of another infection taking a foothold.”
AD
The steroid could add to a tiny but growing arsenal of drugs supported by evidence being used against covid-19. Remdesivir, an antiviral drug, was given emergency-use authorization in the United States after a trial showed it reduced the length of hospitalization. Several human tests have begun of monoclonal antibody drugs, which many experts hope could provide a powerful weapon against the disease.
Physicians are hopeful that if a second wave of coronavirus infections begins to surge in the fall, they will have more therapies and knowledge to save people’s lives.
Nick Cammack, covid-19 therapeutics accelerator lead at Wellcome, the philanthropic organization that helped pay for the British research, called the results a “breakthrough” in a statement and said dexamethasone should be used around the world for critically ill patients but added this was only the beginning.
“This is extremely promising news and a significant step forward, but we still have a long way to go,” he said. “To end this pandemic, we still need better diagnostics to detect, medicines to treat and vaccines to prevent covid-19.”
Coronavirus: What you need to read
The Washington Post is providing some coronavirus coverage free, including:
Updated June 16, 2020
The latest: Live updates in the U.S.
Coronavirus maps: Cases and deaths in the U.S. | Cases and deaths worldwide | Which states are reopening
Have you been hospitalized for covid-19? Tell us whether you’ve gotten a bill.
25 Comments
Today’s Headlines
The most important news stories of the day, curated by Post editors and delivered every morning.
Enter your email address
By signing up you agree to our Terms of Use and Privacy Policy